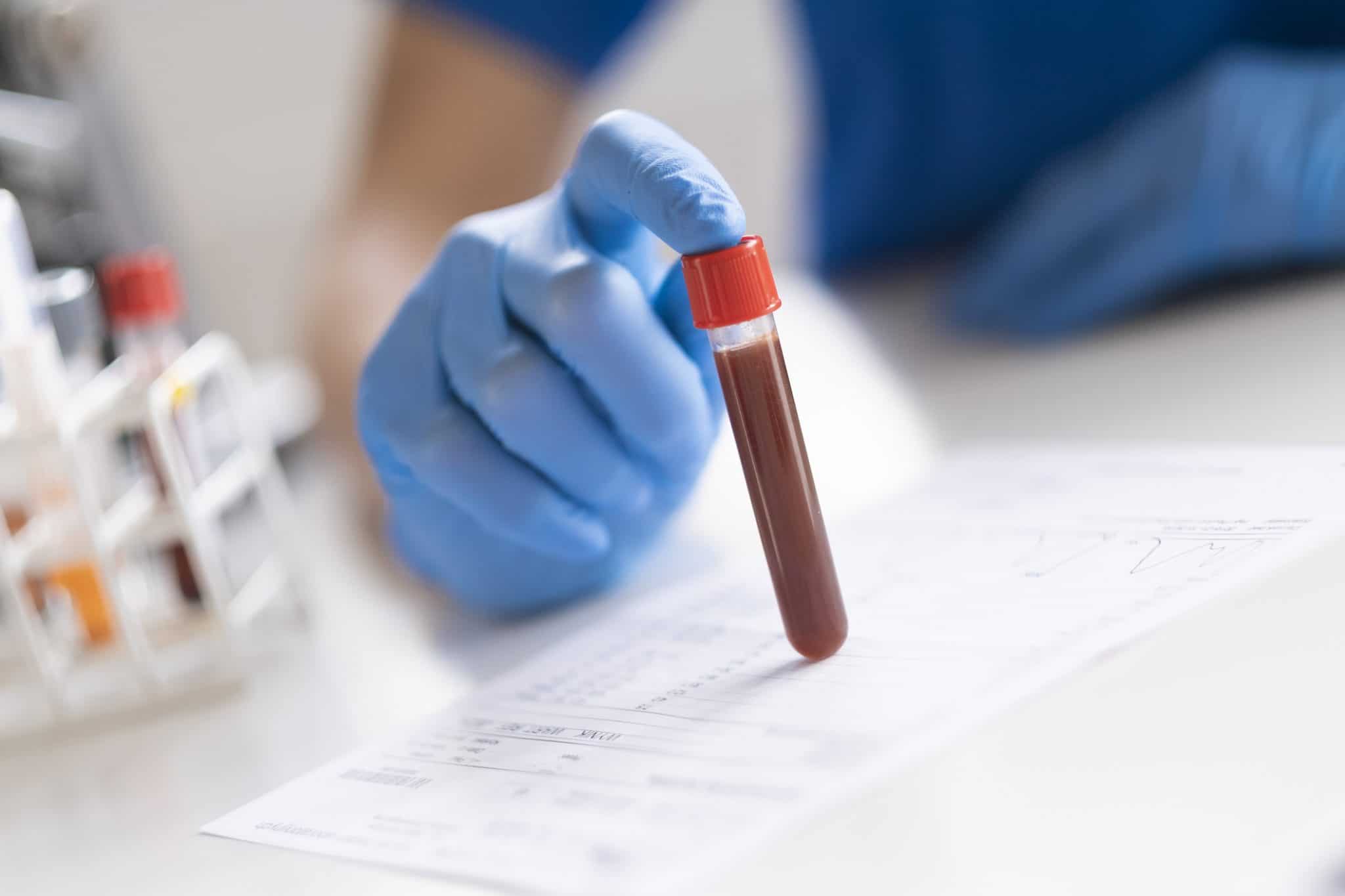
Recently, Roche’s antibody test has been granted emergency approval from the U.S. Food and Drug Administration in order to control and prevent further spread of the coronavirus infection as well as look for any developments of antibodies and immunity against the COVID-19.
The head of diagnostics of the company, Thomas Schinecker, has stated that Roche is making efforts to increase its production of the antibody test from fifty million to around more than one hundred million every month by the end of 2020.
Currently, such tests are needed by companies, governmental institutions, and even small businesses for preventive measures as it will allow them to know any worker who may have COVID-19, who may be more vulnerable to developing it, or who may have a stronger immune system to overcome it.
The provided testing may also help in developing further ways to control the pandemic and make policies that may help in ending the nationwide lockdowns as soon and as safely as possible.
Roche, which has also developed diagnostic molecular tests for the detection of active coronavirus infection, has claimed that its new antibody test has a higher sensitivity and specificity rate.
More specifically, the antibody test has been seen to have a sensitivity rate of one hundred percent and a specificity rate which is more than ninety-nine percent. This means that the test is unlikely to show the wrong results where other tests have a problem of showing false positive and negative tests.
The problem with the wrong test results is that they can significantly increase the risk of further spread. For instance, a false positive test may mistakenly show a person has an immune system strong enough for COVID-19.
RELATED: ISIS Terrorist Attack Continue During COVID-19 Pandemic
In the new test, the blood is drawn intravenously. Health experts at Roche have explained that this is because intravenous draws are much more reliable and accurate in comparison with tests that require drawing blood through finger-pricks.
While the Roche test has gained FDA approval, a number of other companies have also developed similar antibody tests and claimed to have an equally high rate of sensitivity and accuracy.
Some of the companies with tests similar to Roche’s include Becton Dickinson, U.S.-based Abbott Laboratories, and Italy’s DiaSorin.
According to a report, a number of distributors have entered the drug market recently because of the rising demands for coronavirus testing.
However, not all of them are experienced or are as competent as other well-known companies but have still managed to join them which shows the lack of regulation in the US drug market.
The primary reason for the high demand comes with the rising number of cases in the front line health care workers. Since there is a shortage of personal protective equipment and workers are exposed to a high-risk environment, more and more of them are contracting the virus.
In addition, the exposed workers are also likely to infect other people they may come in contact with including friends and family outside of hospital settings or other patients within the hospital.
Therefore, high-accuracy tests for immunity and coronavirus detection are needed at a very high number. Roche’s antibody test is expected to meet these demands as soon as possible.
Till now, the company has not revealed the price for its antibody test but has ensured that it will be available at the same rate around the world. Thomas Schinecker has also reportedly said that there is still a lot to learn about the novel coronavirus.
While it has been established that antibodies help in fortifying immunity against infections, there is a need for scientific proof in the case of COVID-19 as the virus’s behavior is different from other pathogens.