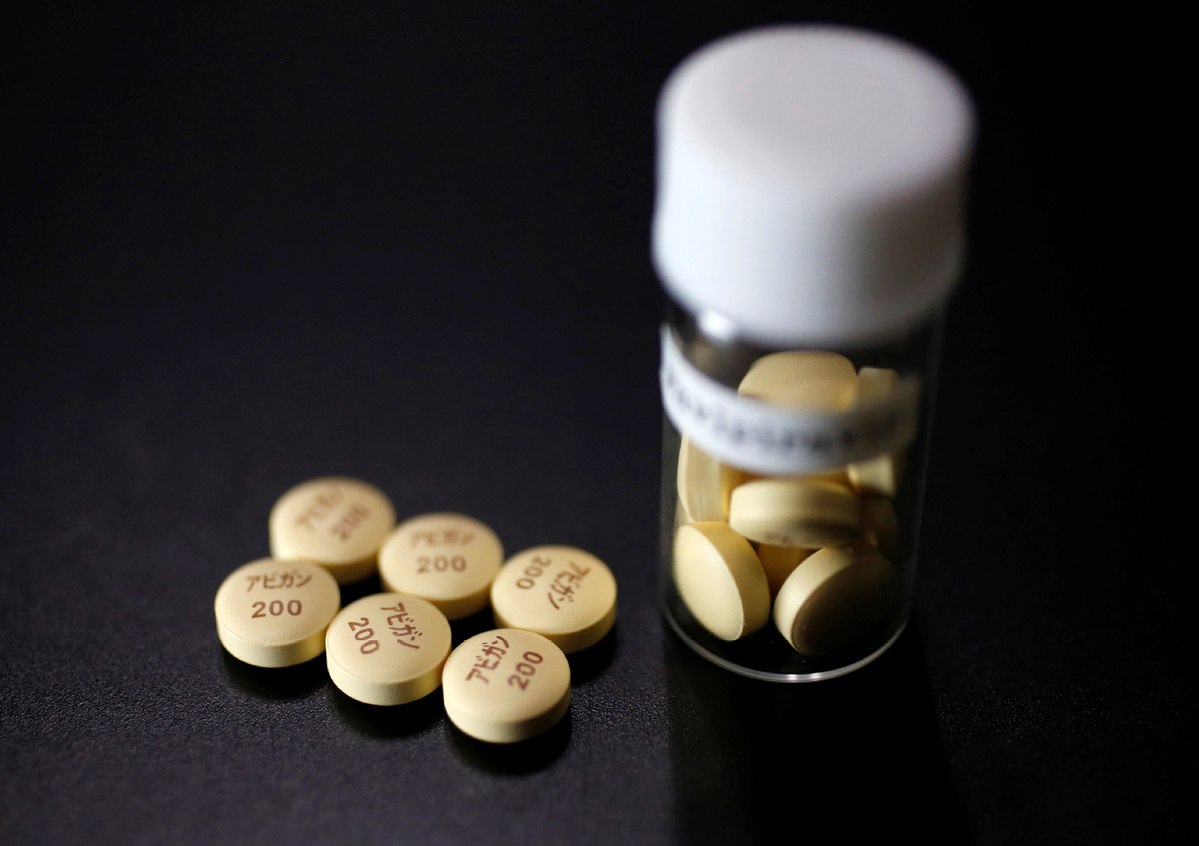
The novel coronavirus remains a threat all around the world since a vaccine is not available in the market yet. An antiviral drug against the virus has been approved in Russia and will be given to the infected patients from the coming week. The first approved antiviral drug for COVID-19 will prove to be a game-changer since the lockdown has caused an economic crisis in various countries.
Also read- Exercise Can Cut Down the Risk of Maternal Depression During Coronavirus Pandemic
The head of the RDIF sovereign wealth fund of Russia said that around 60,000 patients will be given the drug per month. The hospitals will start administrating the drug from June 11.
There is no vaccine available for the coronavirus yet and the first approved antiviral drug for COVID-19 in Russia has not been through consensus in the scientific community around the globe.
The drug will be sold by the name Avifavir. This drug passed the clinical trials after which the government approved its use. It is approved by the health ministry of Russia and the first effective treatment against the lethal coronavirus.
Kirill Dmitriev is the head of RDIF who said that the drug went through a clinical trial that involved 330 participants. The drug was successful in treating most of the coronavirus patients in four days.
He said the drug will undergo more clinical trials and the current trial will be concluded in a few days. After that, the drug will be available for use by the coronavirus patients. Russia’s health ministry approved the drug against the virus through an accelerated process of approval. The manufacturing of the drug was already started in March.
He also said that the drug is believed to be a game-changer. This first approved antiviral drug for coronavirus will help reduce the number of people who get severely ill due to the disease which will eventually reduce the stress on the health care system. The drug eradicates the virus from the body in 10 days in almost 90% cases of the coronavirus.
The economy of Russia is believed to fall back out of crisis after the approval of this drug. But a vaccine is still needed to counter the virus and social distancing must be ensured along with other preventive measures to control the outbreak.
The fatality rate due to Covid-19 in Russia is much low than in other countries with 4,693 reported deaths. The number of cases is third highest in the world with 405,843 coronavirus cases which are less than the United States and Brazil.
Also read- New Spit Tests Can Help Detect Cancer In Women At Home
The drug does not have severe side effects but it should not be used by pregnant women. The first approved antiviral drug will be available in the form of a tablet which is easier to take by the patients. Hence, the patients will not have to spend a lot of their time in the hospital and there will be a lesser risk of spreading the virus. Dmitriev said that the drug will be effective in the case of patients with mild symptoms.
The trials for the drug were funded by RDIF which holds half of the share in ChemRar, the manufacturer of the drug.
Dmitriev said that the country will look towards exporting its first approved antiviral drug for COVID-19 after the medical needs of Russia are covered.